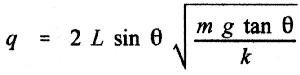Coulomb's Law
Introduction
Coulomb's law describes electrostatic forces.
Electrostatic force is an important force because it is the force that keeps
molecules together.
The first verification of the law of electrostatic
forces was made by the French engineer Charles Augustus Coulomb (1736-1806).
Using hairs and wires he constructed a torsion balance similar in design
to Cavendish's experiment. The amount of torsion required to bring a charged
pithball within various distances of another pithball allowed for the calculation
of k, the electrostatic constant.
Materials: ring stand with wooden dowel, two pith balls, string,
fur, charging rod, protractor, electronic balance, ruler
Methods
1. Obtain an average mass for the two pith balls and measure the length of
the string (L) to the center of the pith ball.
2. Suspend the two pith balls from the wooden dowel attached to the ring
stand such that their strings have a common point of origin.

3. Using the fur, charge the rod negative and transfer the charge to the
two pith balls.
4. Measure the angle (= 2 q in the
equation below) that the two
strings make with each other at the point of origin.
Analysis
1. Sketch a free body diagram for each pith ball showing all forces acting
on each while suspended by the electrostatic force.
2. Using the value of electrostatic constant (k) and the problem we solved
in class, determine the charge (q) on each pith ball.
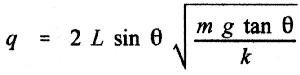
3. Using dimensional analysis, verify that the units in your equation condense
to a coulomb.
4. Calculate the number of excess electrons on each pith ball while each
was suspended.
5. Why were equal sized pith balls used?
6. If an ampere is defined as the flow of electrons in Coulomb/second (C/s),
how many amps would each pith ball generate if it flowed in a wire. Compare
to the current consumed by a television set.
Back to the Brockport High School Science Department